How to Handle the Flu While Pregnant
Getting the flu is never fun—but it can be especially tough when you’re expecting. Pregnancy brings lots of changes to the immune system to aid baby’s development, meaning illness can hit a little harder during those nine+ months. While you’re at greater risk of coming down with a more severe case of the flu during pregnancy, luckily there’s lots that can be done to ensure both your and baby’s health. Here, experts break down what to know about getting the flu while pregnant and how to safely treat it.
The flu is a contagious respiratory illness caused by influenza viruses. It’s transmitted through droplets in the air when infected people cough, sneeze or talk from as far as six feet away, says infectious disease specialist Richard R. Watkins, MD, a professor at the Northeast Ohio Medical University. “Someone might also get the flu by touching a surface or object that has the flu virus on it and then touching their own mouth or nose,” he adds. The flu can lead to symptoms ranging from mild to severe and, in rare cases, can be life-threatening.
Differentiating between the flu and COVID-19
While symptoms from the flu in pregnancy will likely be more severe than a cold, it can be harder to tell the difference between the flu and COVID. According to the Centers for Disease Control and Prevention (CDC), it’s challenging to differentiate from symptoms alone. The main difference between the two comes with the onset of symptoms: While symptoms from the flu usually start one to four days after exposure, symptoms from covid can start up to 14 days after exposure. But of course, it’s tough to tell exactly when you were exposed to illness. The best way to get properly diagnosed is to get tested by a medical professional.
The odds of contracting the flu during pregnancy are the same as when you’re not expecting, but the level of severity changes, says Michael Cackovic, MD, a maternal-fetal medicine physician at the Ohio State University Wexner Medical Center. According to the American Pregnancy Assocation (APA), pregnancy naturally increases heart rate, decreases lung capacity and suppresses the immune system, which can lead to more severe illness and increase the risk of developing serious complications, like pneumonia or bronchitis. American College of Obstetricians and Gynecologists (ACOG) says hospitalization is more likely if you get the flu while pregnant than when you’re not pregnant. “The flu is actually four to five times more dangerous in pregnancy,” Cackovic says, adding that it can also be fatal in rare cases.
How the flu during pregnancy affects baby
Studies have found that having the flu while pregnant can also potentially affect baby. A 2013 and 2019 review found that fevers during early pregnancy were associated with increased risks of birth defects, such as neural tube defects and heart anomalies. According to Cackovic, there’s a higher incidence of birth defects in babies whose moms had the flu in the first trimester compared with those who didn’t. This is likely due to the fever that so often accompanies the flu, he explains. Pregnant women who get the flu are also more likely to experience preterm labor, the ACOG notes.
While this sounds scary, it’s important to note that the flu really only affects baby if the mom-to-be becomes very ill, says Cynthia Flynn, MD, a Florida-based ob-gyn with JustAnswer. Plus, thanks to the flu vaccine, there’s an easy way to protect both yourself and baby (more on this below).
The classic flu symptoms remain the same regardless of pregnancy. However, if you think you’re coming down with the flu, it’s important to let your doctor know as soon as possible. Early treatment can help stave off the worst of the flu symptoms and get you on the road to recovery faster. Here are the pregnancy flu symptoms to look out for, according to the APA:
- A cough or shortness of breath
- Sore throat
- Fatigue (beyond your norm during pregnancy)
- Fever
- Chills
- Headache
- Muscle or body aches
- Runny nose
- Loss of appetite
- Diarrhea
- Vomiting
The APA also urges moms-to-be to call their doctor right away or head to the emergency room if they experience any of the following symptoms:
- Difficulty breathing
- Chest pain or pressure
- Sudden dizziness
- Confusion
- Severe or continuous vomiting
- High fever that isn’t responding to Tylenol
- Decreased fetal movement
How the flu is diagnosed
If you suspect you have the flu, your doctor will probably perform what’s known as a rapid flu test in their office, which generally involves a nose or throat swab. It takes about 15 minutes to get the results, but it’s not 100 percent accurate. “The rapid flu test is positive in about 50 to 70 percent of people with the flu,” Watkins says. Though it’s not totally accurate, doctors are usually able to make a diagnosis based on symptoms alone.
How long does the flu last?
An uncomplicated course of the flu usually lasts anywhere from three to seven days, though a cough and general feeling of being sick can last two weeks or more. However, Watkins says that during pregnancy these symptoms may last a little longer, especially if complications of the flu, such as pneumonia, come up. Ultimately, like so many other aspects of pregnancy, the length of time it takes to get back to normal will vary from person to person.
When it comes to the flu, there isn’t much doctors can do to treat influenza viruses. This is because they’re viral infections, meaning they won’t react to antibiotics the way bacterial infections will. Viral infections often need to run their course before they go away, but the good news is there are ways to shorten the time you spend feeling sick.
Taking cold and flu medicine while pregnant
If you’re coming down with the flu in pregnancy, odds are you’ll be taking a trip to your doctor to get tested and come up with a treatment plan. If you see your doctor within the first 48 hours of developing symptoms, they can prescribe a pregnancy-safe antiviral medication (like Tamiflu) that can shorten the length of the flu by a day and potentially help lower your risk of developing complications, Watkins says. Since a flu-related fever in early pregnancy can be dangerous for baby, it’s important to get a spiking temperature under control. Luckily, Tylenol (acetaminophen) is considered safe for treating a fever during pregnancy, Flynn says.
Wondering if it’s safe to take over-the-counter cold and flu medicines while pregnant? While you likely won’t need these if your doctor prescribes you antiviral medication, cold and flu medicine from Tylenol (acetaminophen), Robitussin and Benadryl is usually safe, Flynn says. “Many others may be safe, but you should check with your doctor first,” she adds.
Home remedies for the flu during pregnancy
Alongside any medication your doctor recommends, there are some home remedies that can help make you more comfortable and get you feeling better faster.
-
Drink lots of fluids. Hydration is extremely important during pregnancy, and even more so if you’re sick with the flu. Make sure to drink plenty of liquids to help strengthen your body’s immune response to the virus. One good option? Bowls of hot soup or broth, which can help loosen chest congestion, says Julie Lamppa, CNM, APRN, a nurse midwife at the Mayo Clinic. Studies also suggest that chicken soup may have a mild anti-inflammatory effect, helping to ease respiratory infections. Another easy way to break up chest congestion, Lamppa suggests, is adding lemon and honey to a cup of hot water.
-
Run a humidifier. The air in your home can be especially dry in the winter. Along with fluids, plugging in a cool mist humidifier to add some moisture to the room can also help ease chest congestion.
-
Use saline nasal sprays. As if chest congestion wasn’t bad enough, nasal congestion is another uncomfortable symptom of having the flu in pregnancy. Luckily, Lamppa says over-the-counter saline sprays and drops are a pregnancy-safe way to help clear out your nasal passages.
-
Gargle with salt water. It may not be the tastiest of home remedies for flu, but if you’re struggling with a sore throat, Lamppa says mixing up a gargle of warm water and salt can help ease the pain.
-
Rest. This one may seem like a no-brainer, but it can be hard to do with all the to-do’s on your plate. Being sick (and pregnant!) takes a lot out of you. Resting allows your body to focus on fighting off the illness, Watkins says. Stay home from work, have a loved one watch any little ones—do what you need to do to ensure you can prioritize your recovery for as long as you need.
No one likes getting sick, especially with the flu. While you can’t control the cold and flu season, you can take preventative measures against catching the flu in pregnancy. Luckily, thanks to the COVID-19 pandemic, many people now keep preventative practices top of mind. Some ways to stay healthy include:
- Frequent and thorough handwashing (especially after going out in public)
- Distancing yourself from people currently sick or displaying symptoms
- Not touching your eyes, nose or mouth
- Avoiding shaking hands with others
- Wearing a mask in indoor and crowded spaces
- Getting the flu shot
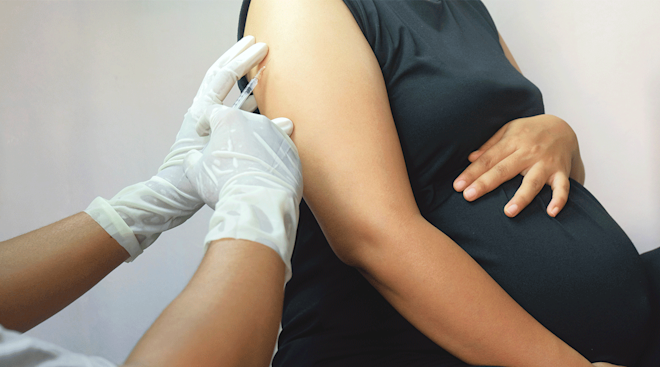
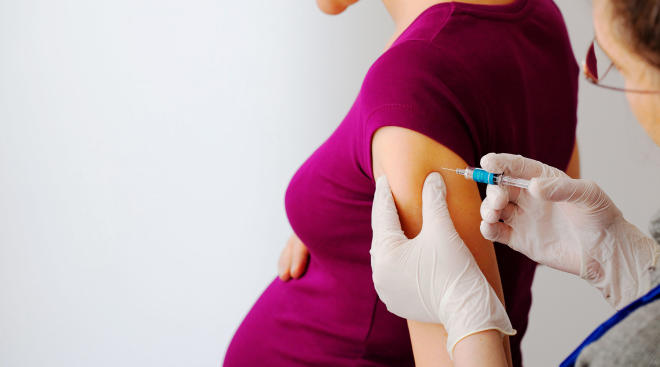
Getting the flu shot while pregnant
Doctors and government health agencies strongly encourage women to get the flu shot during pregnancy. The vaccine changes from year to year, as it’s designed to protect against the influenza viruses that research suggests will be the most common that season. And experts agree the benefits of getting the flu vaccine during pregnancy are numerous. According to the CDC, the flu vaccine in pregnant women has been proven to reduce the risk of infection by approximately 50 percent, and it reduces the risk of hospitalization by an average of 40 percent. Even if you do wind up getting the flu, if you’ve been vaccinated, you’re less likely to develop serious complications associated with the illness, Watkin says.
Getting the flu shot while pregnant also offers big benefits for baby. Infants under 6 months are too young to get the flu vaccine, but the CDC notes that getting the vaccine during pregnancy can pass protective antibodies to baby and offer protection from a flu infection for several months after birth.
Worried that the flu vaccine will make you sick? Don’t be. “It’s impossible to get the flu from the flu shot because there’s no live flu virus in the vaccine,” Cackovic says. (Both the ACOG and CDC note that the nasal spray vaccine, which does contain the live virus, shouldn’t be given to moms-to-be.) Other than mild pain or tenderness where the vaccine was injected, Watkin says there aren’t any notable side effects to getting the flu shot while pregnant, and you can get it at any point during pregnancy.
Getting the flu while pregnant can be scary, but know there are several preventative measures you can take to keep both you and baby healthy. And if you do end up getting sick, don’t stress. Call your doctor, and they’ll come up with the best treatment plan to help you feel better soon.
About the experts:
Richard R. Watkins, MD, FACP, FIDSA, is an Ohio-based infectious disease specialist and professor at Northeast Ohio Medical University. Watkins has more than two decades of experience. He received his medical degree from American University of the Caribbean School of Medicine, completed his internal medicine residency at Allegheny General Hospital and finished his infectious diseases fellowship at Hahnemann University Hospital.
Michael Cackovic, MD, is a maternal-fetal medicine physician at the Ohio State University Wexner Medical Center. He completed his medical degree at MCP Hahnemann University College of Medicine, his residency at St Luke’s Hospital and Health Network and his fellowship at Yale New Haven Hospital.
Julie Lamppa, CNM, APRN, is a nurse midwife at the Mayo Clinic and clinical instructor in the Department of Obstetrics and Gynecology at the Mayo Clinic College of Medicine and Science. She served as a labor and delivery nurse for 15 years prior to earning her master’s degree in midwifery in 2009.
Cynthia Flynn, MD, is a board-certified ob-gyn based in Florida with over 20 years of experience. She is also an expert with the online platform JustAnswer. She received her degree from the Michigan State University College of Human Medicine.
Please note: The Bump and the materials and information it contains are not intended to, and do not constitute, medical or other health advice or diagnosis and should not be used as such. You should always consult with a qualified physician or health professional about your specific circumstances.
Plus, more from The Bump:
Navigate forward to interact with the calendar and select a date. Press the question mark key to get the keyboard shortcuts for changing dates.